Electric dipole and its physical significance
A pair of two equal and opposite charges separated by some distance is called an electric dipole.
Example:Two charges + q and -q separated by a small distance 2l constitute an electric dipole as shown in figure.
-
The distance (2l) between two charges is known as dipole length.
-
Total charge of an electric dipole is zero but electric field of a dipole is not zero.
Electric dipole Moment (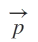 )
)
Electric dipole moment of an electric dipole is defined as the product of the magnitude of either charge and the dipole length.
-
Dipole moment is a vector quantity.
-
The direction of dipole moment is from negative to positive charge.
-
S.I. Unit of dipole moment is coulomb metre (Cm).Another unit of dipole moment is debye.
-
Q: What is Ideal dipole?
Ans.An ideal dipole is that whose size tends to zero (i.e., l → 0) and charge tends to infinity (q → ∞) such that dipole moment p remains constant.
Q: What is the
physical significance of an electric dipole?
Ans.Electric dipoles are very important for the study of the electrical behaviour of matter. As we know that matter consists of molecules which are electrically neutral.These molecules may be polar or non polar.
Polar molecules:
The molecules are said to be polar,if the centre’s of protons (positively charged) does not coincide with electrons(negatively charged).These molecules do have permanent dipole moment but in random direction so their net dipole moment becomes zero. When electric field is applied to them, they align with the direction of electric field to give net dipole moment. This process is called as polarisation.
Non polar molecules :
In non polar molecules, the centre’s of positive charges and of negative charges lie at the same place.These molecules have zero dipole moment, but when electric field is applied to them, their positive and negative charges displace to give induced dipole moment.
Therefore, electric dipole has great physical significance in electrical properties.
