Nucleus
-
Positive charge of an atom is concentrated in the nucleus due to positively charged particles known as protons. Proton is a positively charged particle.
-
The number of protons in the nucleus is called atomic number (Z) of the atom.
-
The sum of the number of protons (Z) and the number of neutrons is called mass number (A).
A = Z + N
-
The specific nucleus of an atom is represented by
.X denotes the chemical symbol of the element whose atomic number is Z and mass number is A.
-
Proton and neutron are collectively called as nucleons.
|
Neutrons
|
|
|
Discovery
|
Neutron was discovered by Chadwick in 1932.
|
|
Properties
|
|
|
Isotopes
|
|
|
Isobars
|
|
|
Isotones
|
The atoms of the elements whose nuclei have the same number of neutrons are called isotones.
|
|
Mirror isobars
|
Two nuclei with same mass number but whose atomic number differ by 1 are called mirror isobars.
|
|
Isodiaphers
|
Nuclides having the same neutron excess, i.e. number of neutrons minus number of protons.
|
Atomic mass unit (a.m.u.)
1 a.m.u. is equal 1/12th mass of Carbon-12 atom.
Relation between a.m.u. and energy or energy equivalent to 1 a.m.u.
Size of Nucleus
The radius of a nucleus depends only on its mass number (A) according to the relation
Nuclear Density
The mass per unit volume of a nucleus is called nuclear density (ρ).
-
Nuclear density is independent of mass number of an atom.Therefore,nuclear density of all atoms is same.
-
Matter is not uniformly distributed inside the nucleus.
-
Density of a nucleus is maximum at its centre and decreases as we move outwards from the nucleus.
Nuclear Forces
-
It is the most strong force in the universe and it acts only between the nucleons.
-
This force was postulated by a Japanese physicist Yukawa.
-
Yukawa predicted that the nuclear forces arise due to the exchange of particles known as π-mesons between the nucleons.
-
π-mesons is a fundamental particle whose mass is 270 times the mass of an electron.
Properties of Nuclear force
1. It is short range force.
2. It is charge independent.
3. It is the strongest force in nature.
4. It is spin dependent.It has been observed that the nuclear force between nucleons having parallel spins is greater than the force between nucleons having anti-parallel spins.
5. It is a saturated force.A nucleon can attract only the nearest neighbours and has no influence on other nucleons.
6. It is non-central force.
7. It is an exchange force.
8. Nuclear force has a small component of repulsive force.
The variation of nuclear force ( in blue curve) with distance between the nucleons is shown in figure below:
-
When the distance between two nucleons is large,the nuclear force is attractive and small in magnitude.
-
As the distance between them decreases,this force goes on increasing till it becomes maximum at a certain distance of separation.
-
With further decrease in distance between the nucleons,this force starts decreasing rapidly till it becomes zero.
-
When the distance between two nucleons decreases further,repulsive component of nuclear force increases rapidly which avoids the collapsing of the nucleus.
MASS DEFECT AND BINDING ENERGY
Mass defect : The difference in mass of a nucleus and its constituents is called the mass defect of that nucleus.
Consider a nucleus of mass M having Z protons and (A – Z ) neutron.Then,
Packing fraction :Packing fraction (f) of an atom is the difference between mass of nucleus and its mass number divided by the mass number.
-
Packing fraction measures the stability of a nucleus.
-
Smaller the value of packing fraction,larger is the stability of the nucleus.
Binding Energy :The total energy required to disintegrate the nucleus into its constituent particles (i.e. nucleons) is called binding energy of the nucleus.
Binding energy is basically the energy required to hold the nucleons in the nucleus.
B.E. = Δm × 931 MeV
Binding energy per nucleon
It is the average energy needed to separate a nucleus into its individual nucleons.
Binding Energy Curve
It is curve drawn between binding energy per nucleon and mass number as shown in the figure.
(i) Some nuclei with mass number A < 20 have large binding energy per nucleon than their neighbouring nuclei. For example,  have more binding energy per nucleon than their neighbours. So these nuclei are more stable than their neighbours.
have more binding energy per nucleon than their neighbours. So these nuclei are more stable than their neighbours.
(ii) For A > 40, binding energy per nucleon increases gradually till it attains a maximum value 8.8 MeV corresponding to Iron 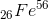 nucleus. Thus, Iron is a stable element.
nucleus. Thus, Iron is a stable element.
(iii) For nuclei having A > 56, binding energy per nucleon gradually decreases. For uranium (A =238), one of the heaviest natural element, the value of B.E/nucleon drops to 7.5 MeV.
Significance of binding energy per nucleon
Binding energy per nucleon determines the stability of a nucleus i.e.,stability of a nucleus is proportional to the binding energy/nucleon.
If binding energy per nucleon of a nucleus is less, the nucleus is less stable whereas the nucleus is more stable if its binding energy per nucleon is higher.
-
The binding energy per nucleon has low value for both the light and heavy nuclei. So they are unstable nuclei.
-
The intermediate nuclei have large value of binding energy per nucleon, so they are more stable.
-
Binding energy per nucleons of light nuclei having mass number A = 4, 8, 12, 16 and 20 is more than the neighbours. This shows that these nuclei are more stable than their neighbours.
Note: Binding energy of a nucleus has nothing to do with the stability of the nucleus.
