In 1895, Wilhelm Konard Roentgen discovered X-rays while working with a discharge tube. X means unknown.
X-rays are electromagnetic radiation of very short wavelength and high energy which are emitted when fast moving electrons or cathode rays strike a target of high atomic mass.
Production of X-rays
Coolidge X-ray tube : Coolidge X-ray tube used to produce X-rays is shown in figure below
X-rays are produced when energetic (fast moving) electrons strike a target such as a metal piece. When electrons collide with the atoms of solid, they loose their kinetic energy which is converted into radiant energy in the form of X-rays. The figure shown essential features of a modern X-ray tube developed by Coolidge.
Coolidge’s X-ray tube consists of a glass bulb exhausted to nearly perfect vacuum. The cathode C is the source of electrons by using a heated filament getting supply from battery B . The anode is made of solid copper bar A . A high melting metal like platinum or tungsten is embedded at the end of copper rod and serves as target T . A high d.c. voltage V(50 kV) is maintained between cathode and anode.
The energetic electrons strike the target T and the X-ray are produced. Only about 1-10% of the energy of the electrons is converted to X-rays and the rest is converted into the heat. The target T as a result becomes very hot and therefore should have high melting point. The heat generated is dissipated through the copper rod and the anode is cooled by water flowing through the anode.
1. An increase in the filament current increase the number of electrons it emits. Larger number of electrons means an intense beam of X-rays is produced. This way we can control the quantity of X-rays i.e. Intensity of X-rays.
2. An increase in the voltage of the tube increase the kinetic energy of electrons (eV = 1 / 2mv²) .When such highly energetic beam of electrons are suddenly stopped by the target, an energetic beam of X-rays is produced. This way we can control the quality of X- rays i.e. penetration power of X-rays .
3. Based on penetrating power, X-rays are classified into two types. HARD-X-rays and SOFT-X-rays. The first one having high energy and hence high penetration power are HARD-X-rays and another one with low energy and hence low penetration power are SOFT-X-rays.
X-ray Spectra
It has been observed that there are two types of X-rays, (i) Continuous X-rays and (ii) Characteristic X-rays. Therefore, the spectrum of X-rays is of two types :
(i) Continuous X-rays spectrum
(ii) Characteristic X-rays spectrum
The continuous spectrum has a minimum wavelength limit, an energy distribution curve with the maximum intensity at λmin × 3/2 . The upper limit of wavelength i.e. the minimum photon energy has no theoretical, limit, it is limited by the ability of the apparatus to detect it. When the potential difference applied was changed, λmin and the distribution of the curve also varied.
Characteristic X-rays were characteristic of the material of the anode and they were emitted only when the potential difference across the tub exceeded a certain threshold, this threshold being a characteristic of the material. With the change in potential unlike the continuous spectrum, the wavelength of characteristic X-rays did not change.
Properties of X-rays
(1) X-rays are electromagnetic waves of very short wavelengths. The wavelength of X-rays ranges from 0.001 nm to 1.0 nm.
(2) They are highly penetrating rays and can even pass through the solids which are opaque to ordinary light.
(3) They affect photographic plate more effectively than the ordinary light.
(4) They cause fluorescence in certain substances like zinc sulphide, barium platinocyanide etc.
(5) They have a destructive effect,on the living tissues.
(6) They ionize the gases through which they pass.
(7) They eject the electrons from certain metals when they fall on them That is, they cause photo-electric effect.
(8) They travel in straight lines with the speed of light (i.e. 3 x 108 m s-1).
(9) X-rays are diffracted like ordinary light.
(10) X-rays can be polarised.
Moseley’s Law and Atomic Number
Moseley in 1913, studied the characteristic spectra of many elements by using these elements as the target in the X-ray tube. He observed that
(i) the characteristic X-ray spectra of different elements are similar to each other in the sense that each spectra consists of K, L, M…… series.
(ii) When the square root of the frequency of a given spectral line (say  ) emitted by an element is plotted against the position of the element in the periodic table, a straight line is obtained as shown in figure.
) emitted by an element is plotted against the position of the element in the periodic table, a straight line is obtained as shown in figure.
From these observations, Moseley concluded that there is some basic quantity in the atom which increases in regular steps from one element to the next element. He named this quantity as atomic number. Atomic number is defined as the positive charge on the nucleus of the atom (i.e. number of protons in the nucleus). It is denoted by Z.
Therefore, the figure can be interpreted as : The square root of the frequency of a given spectral line (say 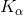 ) emitted. by an element is directly proportional to the atomic number of the element.
) emitted. by an element is directly proportional to the atomic number of the element.
or
which is the Moseley’s law.
Importance of Moseley’s law
1. Moseley’s law (i.e.,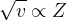 ) provided a guideline to place the elements in the periodic table. Before the investigation of this law, elements were placed in the periodic in the increasing order of the atomic mass of the elements. But Moseley suggested that the characteristic properties of the element are determined by the atomic number (Z) of the element. Hence, after Moseley’s work, the elements were placed in the periodic table in the increasing order of the atomic number of the elements.
) provided a guideline to place the elements in the periodic table. Before the investigation of this law, elements were placed in the periodic in the increasing order of the atomic mass of the elements. But Moseley suggested that the characteristic properties of the element are determined by the atomic number (Z) of the element. Hence, after Moseley’s work, the elements were placed in the periodic table in the increasing order of the atomic number of the elements.
2. Moseley’s law determined the atomic numbers of rare earth elements and their position in the periodic table.
